Cat. #159669
NC9 – Transglutaminase 2 Inhibitor small molecule (tool compound)
Cat. #: 159669
Sub-type: Inhibitor
Availability: Please enquire for quantities and pricing
Application: Has been shown to block TG2 transamidation activity inside cancer stem cells (SCC13 cells) resulting in abolished transamidase activity. Inhibitor blocks EMT and kills cancer stem cells. In mesothiolioma cancer stem cells, inhibition of TG2 reduces migration (invasion), and appears to increase markers of apoptosis.
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Jeffrey Keillor
Institute: University of Ottawa
Tool Details
*FOR RESEARCH USE ONLY (for other uses, please contact the licensing team)
- Tool name: NC9 – Transglutaminase 2 Inhibitor small molecule (tool compound)
- Alternate name: NC9
- Cancers detailed: Colon
- Research fields: Cancer;Cell signaling and signal transduction
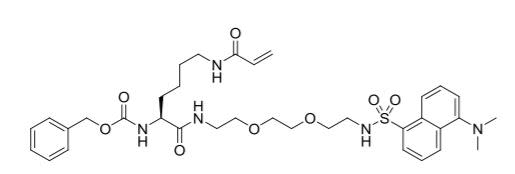
- Molecular formula: C35H47N5O8S
- Tool sub type: Inhibitor
- Primary target: Tissue transglutaminase (TG2)
- Description: Transglutaminases are a family of enzymes that are responsible for mediating the formation of protein crosslinks of several diverse structural proteins (including fibronectin and collagen) through a transamidation reaction between peptide Gln and Lys residues. Tissue transglutaminase (TG2) is a member of this protein family, and is ubiquitously expressed in tissues, primarily found in the cytosol, but is also expressed in the nucleus, membranes, cell surface and extracellularly. TG2 is also...
- Application: Has been shown to block TG2 transamidation activity inside cancer stem cells (SCC13 cells) resulting in abolished transamidase activity. Inhibitor blocks EMT and kills cancer stem cells. In mesothiolioma cancer stem cells, inhibition of TG2 reduces migration (invasion), and appears to increase markers of apoptosis.
- Purpose: Inhibitor
- Selectivity: TG2
- Iupac: benzyl (S)-(1-((5-(dimethylamino)naphthalene)-1-sulfonamido)-10,17-dioxo-3,6-dioxa-9,16-diazanonadec-18-en-11-yl)carbamate
- Cas number: 1352090-52-8
- Molecular weight: 697.84
- Solubility: Soluble in 100 ?M in 5% (v/v) DMSO / aqueous buffers
- Additional notes: NC9 also inhibits FXIIIa. Patent: US20190389814, PCT/IB2017/052162
Handling
- Purity: 697.84 g/mol
- Storage conditions: Dry, dark and at 0 - 4° C for short term (days to weeks) or -20° C for long term (months to years). Aliquot to avoid freeze-thaw cycles. Lasts up to 2 years with proper storage
- Shipping conditions: Dry Ice
Target Details
- Primary target: Tissue transglutaminase (TG2)
Application Details
- Application: Has been shown to block TG2 transamidation activity inside cancer stem cells (SCC13 cells) resulting in abolished transamidase activity. Inhibitor blocks EMT and kills cancer stem cells. In mesothiolioma cancer stem cells, inhibition of TG2 reduces migration (invasion), and appears to increase markers of apoptosis.
References
- Akbar et al. 2017. J Med Chem. 60(18):7910-7927. PMID: 28858494.



![Anti-CAR Whitlow Linker [1C3C3]](https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-300x322.jpg 300w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-280x300.jpg 280w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-954x1024.jpg 954w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-768x824.jpg 768w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025.jpg 1193w)