Cat. #161765
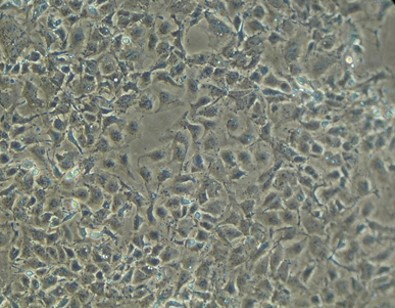
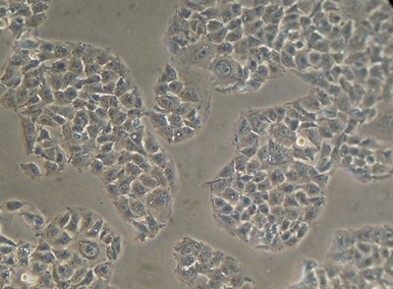
OV-90 Cell Line
Cat. #: 161765
Availability: 8-10 weeks
Organism: Human
Tissue: Derived from malignant ascites of a chemonaive ovarian cancer patient
£575.00
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Anne-Marie Mes-Masson and Diane Provencher
Institute: Centre Hospitalier de L'universite de Montreal
Primary Citation: Provencher et al. 2000. In Vitro Cell Dev Biol Anim. (36): 357–361. PMID:10949993.
Tool Details
*FOR RESEARCH USE ONLY (for other uses, please contact the licensing team)
- Name: OV-90 Cell Line
- Alternate name: OV-90
- Cancer type: Ovarian cancer
- Research fields: Cancer
- Organism: Human
- Gender: Female
- Tissue: Derived from malignant ascites of a chemonaive ovarian cancer patient
- Donor: Grade 3 Stage IV; Mutations: TP53 Exon 6, CDKN2A Exon 2; Pre-treatment
- Crispr: No
- Receptors of note: No
- Description: Tumourigenic epithelial ovarian cancer cell line derived from malignant ascites of a patient never exposed to chemotherapy or radiation therapy. Cell line retains characteristics of the original epithelial ovarian cancers from which it was derived.
References
- Raspaglio et al. 2023. Cancers (Basel). 18,15(8):2361. PMID: 37190289
- Yee et al. 2022. Front Bioeng Biotechnol. 10,10:836984. PMID: 35223797
- Brodeur et al. 2021. Sci Rep. 14,11(1):18183. PMID: 34521878
- Buttarelli et al. 2022. J Exp Clin Cancer Res. 4,41(1):50. PMID: 35120576