Cat. #162283
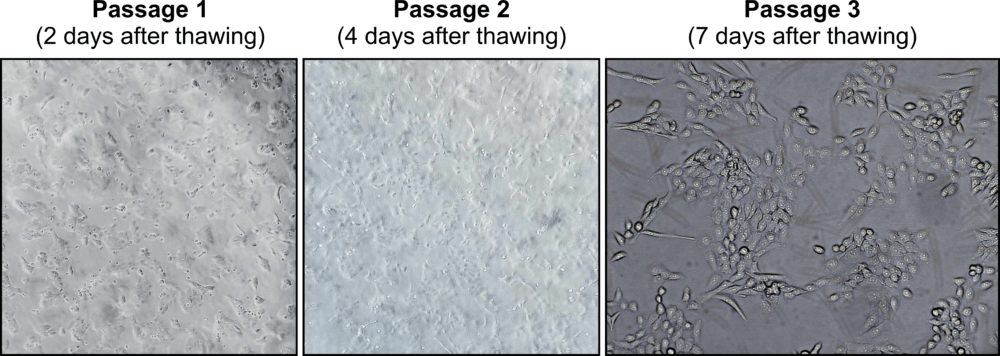
Olaparib-resistant PEO1-OR cell line
Cat. #: 162283
Organism: Human
Tissue: Ovary
Disease: Cancer
£575.00
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Aneta Rogalska, Łukasz Biegała, Agnieszka Marczak
Institute: University of Lodz
Primary Citation: Biegala et al. (2023) Cells, 12(7), 1038, PMID: 37048111
Tool Details
*FOR RESEARCH USE ONLY (for other uses, please contact the licensing team)
- Name: Olaparib-resistant PEO1-OR cell line
- Alternate name: PEO1-OR; PEO1-OR cell line; PEO1-OR cells; olaparib-resistant PEO1-OR ovarian cancer cell line
- Cancer: Gynaecologic cancer
- Cancers detailed: Gynaecologic cancer;High Grade Ovarian;High-Grade Serous Ovarian (HGSOC)
- Parental cell: PEO1
- Organism: Human
- Gender: Female
- Tissue: Ovary
- Disease: Cancer
- Morphology: Epithelial
- Growth properties: Adherent
- Model description: Key mutations determined by whole-exome sequencing using NGS: TP53 (c.731G A; p.G244D); BRCA2 (c.[4964A>T; 4965C>G], p.Y1655L))
- Crispr: No
- Description: The PEO1-OR cell line is a human ovarian cancer cell line with acquired resisatnt to olaparib established from the parental PEO1 high-grade serous ovarian cancer cell line through continuos exposure to step-wise increasing doses of olaparib. PEO1-OR cell line harbours BRCA2 secondary mutation (c.4964A > T) that reverse truncating BRCA2 mutation originating from the parental PEO1 cell line (c.4965C > G; p.Y1655*) and expresses full-length BRCA2 variant (c.[4964A > T; 4965C > G], p.Y1655L). PEO1-OR cells are also less sensitive to ceralasertib (ATR inhibitor) and MK-8776 (CHK1 inhibitor) compared to parental PEO1 cells. PEO1-OR cell line is a powerful in vitro model for studying the molecular and cellular mechanisms of acquiring and overcoming resistance to olaparib in high-grade serous ovarian cancer. This model also allows testing alternate therapies for olaparib-resistant ovarian cancer.
- Application: Cell culture. Resistant cell line models are an essential tool in studying cancer drug resistance mechanisms. The PEO1-OR cell line was used to understand how ovarian cancer cells develop resistance to olaparib. Moreover, The PEO1-OR cell line was used to confirm that combinations of olaparib with the ATR/CHK1 pathway inhibitors act synergistically against ovarian cancer cells. The PEO1-OR cell line can be used to screen for new drugs or drug combinations that can overcome resistance to olaparib and to identify biomarkers associated with drug resistance.
- Production details: PEO1-OR cell line is an olaparib-resistant subline of PEO1 cell line developed by addition of a stepwise increase in olaparib to the growth medium of the parental line.
- Biosafety level: 2
- Additional notes: PEO1-OR cells are grown and subcultured continually in the absence of olaparib. Please be aware that the originating laboratory of the PEO1 cell line acknowledges that PEO1 is both genetically unstable and derived from a heterogeneous population that was already present in the patient at the time of biopsy. This is evident in the literature (Cooke et al., 2010). Genetic differences within the PEO1, PEO4 and PEO6 cell lines suggest that PEO4 and PEO6 are not direct descendants of PEO1 but have diverged from a common ancestor.
Applications
- Application: Cell culture. Resistant cell line models are an essential tool in studying cancer drug resistance mechanisms. The PEO1-OR cell line was used to understand how ovarian cancer cells develop resistance to olaparib. Moreover, The PEO1-OR cell line was used to confirm that combinations of olaparib with the ATR/CHK1 pathway inhibitors act synergistically against ovarian cancer cells. The PEO1-OR cell line can be used to screen for new drugs or drug combinations that can overcome resistance to olaparib and to identify biomarkers associated with drug resistance.
Handling
- Format: Frozen
- Passage number: 37°C
- Growth medium: RPMI 1640 culture medium with 2 mM glutamine and 25 mM HEPES supplemented with 10% heat inactivated FBS
- Temperature: 5% CO2
- Atmosphere: Dry ice
- Shipping conditions: Growth medium (RPMI 1640 culture medium + 2 mM glutamine + 25 mM HEPES + 10% heat inactivated FBS) + 10% sterile DMSO
- Storage medium: Liquid nitrogen, 2×10^6 cells/vial
- Initial handling information: Thaw cells rapidly in a 37°C water bath for up to 2 minutes, until a small amount of ice remains. Slowly add 1 mL of pre-warmed growth medium (37°C) to the thawed cells. Gently reconstitute the cell pellet by pipetting, then transfer the resuspended cells to a centrifuge tube and add 10 mL of pre-warmed growth medium (37°C). Pellet the cells by centrifugation (300×g, 5 min, RT) to remove the storage medium containing DMSO. After the centrifugation, cell pellet should be visible. Aspirate the supernatant, add 1 mL of growth medium, and gently resuspend the cells. Transfer the cells to into the appropriate culture vessel (e.g. T75 flask) containing medium that has been equilibrated in the incubator for a few minutes (37°C, 5% CO2). Optionally, take a small aliquot to assess cell viability. Rock the flask to distribute the cells evenly and place it in the incubator (37°C, 5% CO2). Thawed cells should be plated at high density to optimize recovery. Examine the cells microscopically after 24 hours and sub-culture as necessary.
- Subculture routine: Split sub-confluent cultures (70–90%) every 2 to 4 days using a 0.25% trypsin-EDTA solution. Briefly, remove growth medium, wash cells with PBS without Ca2+/Mg2+, add trypsin-EDTA solution (approximately 1.5–2 mL per 75 cm2 of surface area), place flask in the incubator and leave it for 5–10 minutes (37°C, 5% CO2). Once the cells are detached, add fresh growth medium, and transfer the required number of cells to a new flask. Optionally, after trypsin inactivation, centrifuge cells (300×g, 5 min, RT), resuspend cell pellet in growth medium and passage required number of cells to a new flask. Recommended culture vessel: cell culture treated flasks with filter caps. As a general guide, 70–90% confluent cells should be split in approximately 1:4, 1:6 or 1:12 ratios to reach appropriate confluency after 2, 3 or 4 days. However, splitting ratio may vary depending on cell line handling and culture conditions. The complete formulation of the recommended trypsin-EDTA solution is: KCl (75 mg/L), KH2PO4 (60 mg/L), NaHCO3 (350 mg/L), NaCl (8000 mg/L), Na2HPO4 · 7H2O (90 mg/L), D-glucose (1000 mg/L), EDTA–4Na · 2H2O (380 mg/L), phenol red (10 mg/L), trypsin (2500 mg/L), pH 7.2 to 8.
- Cultured in antibiotics: No
- Mycoplasma free: Yes
- Characterisation tests: Whole-exome sequencing by NGS was used to detect BRCA2 variants. Expression of full-length BRCA2 protein was confirmed by Western Blotting. For details concerning detected variants of genes encoding proteins involved in DNA damage repair, cell cycle regulation, and drug efflux (ABCB1, ATR, BRCA1, CHEK1, H2AX, PARP1, PARG, RAD51, TP53, TP53BP1) see Biegała Ł. et al. PMID: 37048111.
Related Tools
- Related tools: PEO1 cell line; PEO6 Cell Line; PEO14 Cell Line; PEO14 Cell Line; PEO16 Cell Line; PEO23 Cell Line; PEO1-CDDP Cell Line; TO14 Cell Line; PEA1 Cell Line; PEA2 Cell Line
References
- Biegala et al. (2023) Cells, 12(7), 1038, PMID: 37048111