Cat. #160469
Anti-human Neuropilin-1 b1b2 [clone 1A4; mAb1]
Cat. #: 160469
Unit size: 100 ug
Availability: 10-12 weeks
Target: human Neuropillin-1 b1b2 domain
Class: Monoclonal
Application: IHC ; IF ; WB
Reactivity: Human
Host: Mouse
£300.00
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Institute: University of Tartu
Tool Details
*FOR RESEARCH USE ONLY (for other uses, please contact the licensing team)
- Name: Anti-human Neuropilin-1 b1b2 [clone 1A4; mAb1]
- Alternate name: 1A4, mAB1 (mAB1 in studies published in Science)
- Research fields: Microbiology
- Class: Monoclonal
- Conjugation: Unconjugated
- Reactivity: Human
- Host: Mouse
- Application: IHC ; IF ; WB
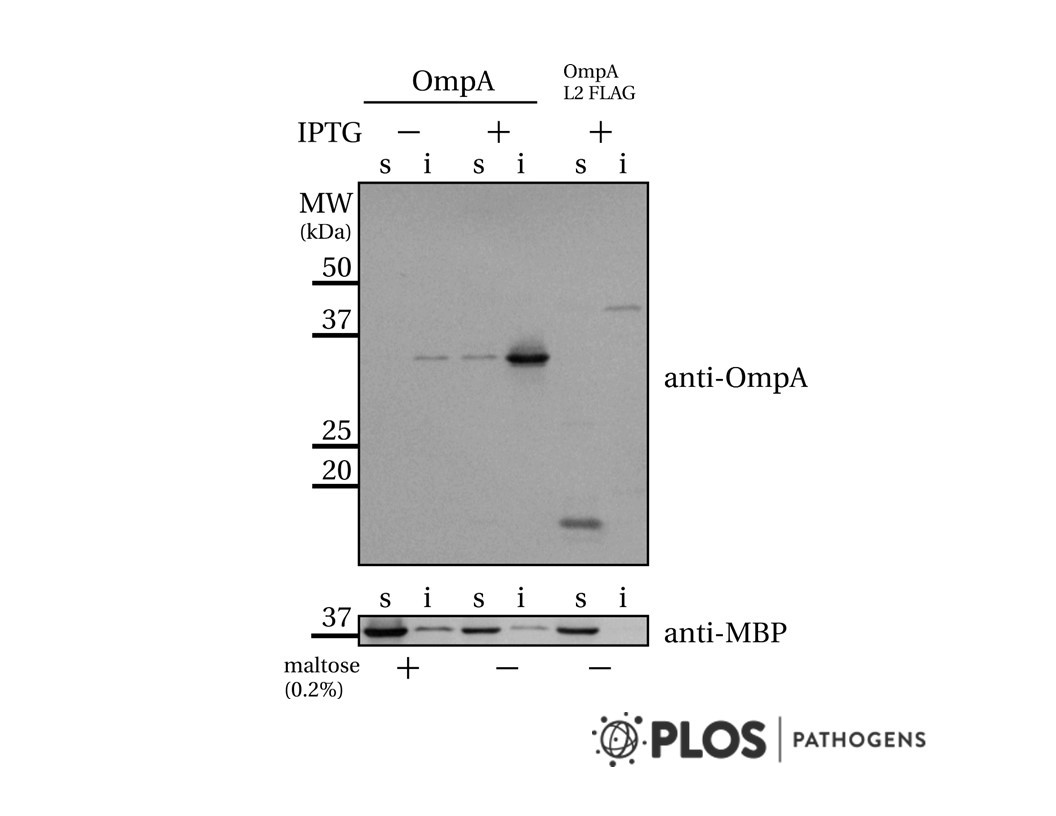
- Description: Monoclonal antibody raised against human NRP-1 b1b2 domain, clone 1A4 (mAB1 in studies published in Science). Partially blocking binding of CendR peptides to b1 domain of NRP-1. In recent years the relevance of CendR pathway for cellular uptake of biological nanoparticles ÄËĂÂĂÂ viruses - has been demonstrated in several independent studies. A direct role of Neuropilin (NRP) in virus entry has been demonstrated for three viruses, the retrovirus Human T-cell lymphotropic virus type 1 (HTLV-1) and the herpes viruses EBV and CMV5-8. In the case of CMV, NRP-2 acts as a receptor only for specific cell types, while in fibroblasts the virus uses a different molecule for entry. Viruses that display CendR peptides on their surface are expected to bind a specific CendR binding pocket on the extracellular domain of NRP. Ample evidence obtained with synthetic and phage-displayed CendR peptides shows that they bind to conserved binding pocket in b1 domain of NRP-1. The inventors have developed a monoclonal antibody that specifically interacts with the CendR binding pocket of the b1 domain of Neuropilin-1 and blocks binding of CendR peptides. This monoclonal antibody has potential applications in the research of SARS-CoV2 in that the antibody is capable to reduce/block the internalisation of SARS-CoV2 into cells, thus to stop viral replication.
- Immunogen: See Xbio/UOT/V/b1b2wt and Xbio/UOT/V/b1b2tm
- Myeloma used: P3X63Ag8.653
Target Details
- Target: human Neuropillin-1 b1b2 domain
- Target background: Monoclonal antibody raised against human NRP-1 b1b2 domain, clone 1A4 (mAB1 in studies published in Science). Partially blocking binding of CendR peptides to b1 domain of NRP-1. In recent years the relevance of CendR pathway for cellular uptake of biological nanoparticles ÄËĂÂĂÂ viruses - has been demonstrated in several independent studies. A direct role of Neuropilin (NRP) in virus entry has been demonstrated for three viruses, the retrovirus Human T-cell lymphotropic virus type 1 (HTLV-1) and the herpes viruses EBV and CMV5-8. In the case of CMV, NRP-2 acts as a receptor only for specific cell types, while in fibroblasts the virus uses a different molecule for entry. Viruses that display CendR peptides on their surface are expected to bind a specific CendR binding pocket on the extracellular domain of NRP. Ample evidence obtained with synthetic and phage-displayed CendR peptides shows that they bind to conserved binding pocket in b1 domain of NRP-1. The inventors have developed a monoclonal antibody that specifically interacts with the CendR binding pocket of the b1 domain of Neuropilin-1 and blocks binding of CendR peptides. This monoclonal antibody has potential applications in the research of SARS-CoV2 in that the antibody is capable to reduce/block the internalisation of SARS-CoV2 into cells, thus to stop viral replication.
Applications
- Application: IHC ; IF ; WB
Handling
- Format: Liquid
- Unit size: 100 ug
- Shipping conditions: Dry ice
References
- Cantuti-Castelvetri et al. 2020. Science. 370(6518):856-860. PMID: 33082293.
- Daly et al. 2020. Science. 370(6518):861-865. PMID: 33082294.

![Anti-human Neuropilin-1 b1b2 [clone 1A4; mAb1]](https://cancertools.org/wp-content/uploads/0c5934f6-fdf5-4ee5-b7ad-82ec2c16e7d8.jpg)

![Anti-CAR Whitlow Linker [1C3C3]](https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-300x322.jpg 300w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-280x300.jpg 280w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-954x1024.jpg 954w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-768x824.jpg 768w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025.jpg 1193w)