Cat. #160936
RLUN005-2 Lung cancer organoid
Cat. #: 160936
Sub-type: Organoids
Unit size: 40-100 uL of cell pellet/vial, 1ml
Organism: Human
Tissue: Lung
£2,500.00
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Medical-Industrial Translational Research Center
Institute: Fukushima Medical University
Primary Citation: Takahashi et al. 2019. Cells 20: 481. PMC6562414
Tool Details
*FOR RESEARCH USE ONLY (for other uses, please contact the licensing team)
- Tool name: RLUN005-2 Lung cancer organoid
- Alternate name: RLUN005-2, RLUN5-2, RLUN5, RLUN005
- Cancer: Adenocarcinoma;Lung cancer;Skin cancer
- Cancers detailed: Adenosquamous carcinoma;Lung;Solid adenocarcinoma;Squamous cell carcinoma
- Research fields: Cancer
- Tool sub type: Organoids
- Organism: Human
- Tissue: Lung
- Gender: Female
- Growth properties: Suspension
- Application: 3D cell culture, High-throughput screening
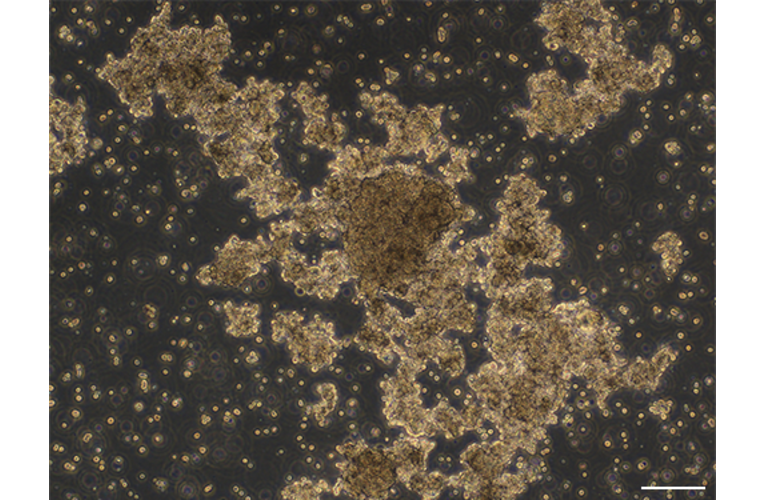
- Description: A series of novel patient-derived organoids (PDOs) have been constructed from different tumor tissue types under the Fukushima Translational Research Project, designated as F-PDO. F-PDOs form large cell clusters with a morphology similar to the original tumor and can be cultured for more than six months. Our comparative histological and comprehensive gene expression analyses have shown that the characteristics of F-PDOs were similar to their source tumors, even after long-term growth in culture conditions
- Biosafety level: 1
Handling
- Format: Frozen
- Passage number: 5
- Growth medium: Cancer Cell Expansion Media plus (Fujifilm Wako Pure Chemical, Ltd.).
- Temperature: 37° C
- Atmosphere: 5% CO2
- Volume: 1 ml
- Storage medium: CELLBANKER 2
- Storage conditions: Liquid Nitrogen
- Shipping conditions: Dry ice
- Characterisation tests: Pathogen and stelility test; Comprehensive gene expression analysis; Cancer panel sequencing; Whole exome sequencing; Anticancer drug susceptibility testing
- Mycoplasma free: Yes
- Biosafety level: 1
Application Details
- Application: 3D cell culture, High-throughput screening
References
- Takahashi et al. 2019. Cells 20: 481. PMC6562414