The research tool: Anti-Omomyc
Cancer is associated with the abnormal growth of cells which proliferate uncontrollably and can metastasize into surrounding tissues. There are over 200 types of cancer and distinct subtypes have been identified. Adding to the challenging complexities of cancer, is tumour heterogeneity, which refers to the differences among tumours of the same type within different patients, among cells within a single tumour, or between a primary and secondary tumour (1). This variability often reduces the efficacy of existing therapies, compromising patient outcomes.
The biotechnological breakthroughs of recent years pushed clinical trials and cancer treatment paradigm to progressively shift from tumour-type centred to precision cancer medicine (2), where the focus is on patient-specific therapies, and challenges like tumour heterogeneity can be overcome through the development of specialized treatments. In contrast, discoveries at the Vall d’Hebron Institute of Oncology (VHIO) support the possibility of a more universal approach, with a single therapeutic option potentially targeting different types of cancer. By taking a universal approach to cancer therapy and targeting the common drivers of cancers across cancer types, this could result in therapies that are universal to cancer patients, but also specific to cancerous cells. The difficulty with this approach, is that these universal targets are often harder to drug, either due to the location or interactions of the target, or because of the universal target’s role in normal cellular function.
CancerTools.org spoke to Dr. Laura Soucek, an investigator at the VHIO and ICREA-born spin-off Peptomyc S.L., and leader of VHIO’s Models of Cancer Therapies Group to find out more.

The contributor
VHIO, ICREA-born spin-off Peptomyc S.L., and leader of VHIO’s Models of Cancer Therapies Group
Targeting a protein altered in many cancer types
Dr. Soucek entered research with one goal in mind – to make a difference in cancer research. To that end, she decided to focus on targeting a protein that was, and still is, considered by many to be “difficult-to-drug” – MYC. The MYC protein sits within the nuclei and acts as a ‘master regulator’ of a variety of cellular functions, including proliferation, differentiation, metabolism, and survival. Due to its multidimensional role, it is critical for MYC to be tightly regulated. Deregulation of MYC leads to altered cellular proliferation and growth, protein synthesis, and metabolism. Additionally, MYC promotes tumour progression through activation of angiogenesis and suppression of the host immune system. However, MYC is a difficult target for cancer therapy. Its location within the nuclei, together with its intrinsically disordered structure and lack of a specific active site, make direct MYC inhibition with traditional strategies challenging. Moreover, all three MYC family members (MYC, MYCN, and MYC) need to be targeted to obtain the most efficient therapeutic impact.
A long road to success
For 20 years, Dr. Soucek has been researching ways to inhibit MYC. In 1998, she designed the dominant-negative form of MYC called Omomyc, as a laboratory tool to study MYC biology. Since then, many milestones have been achieved; first using in vitro systems, transgene expression of Omomyc inhibited MYC activity and reduced proliferation in normal and cancer cells (3). Later, in various animal models of cancer, MYC inhibition by Omomyc exerted remarkable anti-cancer properties, without adverse and irreversible effects (4). Following Omomyc’s successful characterisation as a MYC inhibitor, it is now undergoing clinical development.
”When we turned off MYC in cancer cells using Omomyc, we saw that it had a dramatic therapeutic effect in different types of experimental cancer models. The beauty of it is that, while everybody expected normal proliferating cells to suffer too, they simply slowed down their proliferation, but nothing major happened to them. So, we finally had a tool against cancer that seemed not to cause any severe side effects in normal proliferating tissues. And the other thing that really made me happy was that it appeared to be the opposite of personalised medicine: We had a technology that would be applicable to all types of cancers, so that maybe we didn't need different drugs for each of them, maybe we could use a common one for all cancers and patients.
Dr. Soucek
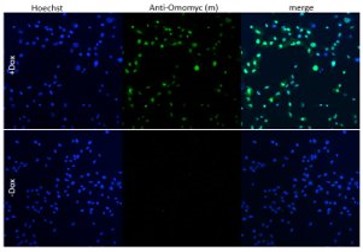
Image: A549 cells with Doxycycline-inducible Omomyc were orthotopically implanted into recipient mice. After treatment with Doxycycline, lung tissue was collected and FFPE lung sections were stained with anti-Omomyc monoclonal [21-1-3].
Omomyc impact
After the promising results obtained in vitro and in vivo, the next step was the conversion of Omomyc to an administrable drug. Dr. Soucek and her team showed that, as an alternative to its use as a transgene, Omomyc could be produced as a recombinant mini-protein. This purified Omomyc mini-protein then showed in Dr.Soucek’s studies that it spontaneously penetrates cancer cells and effectively interferes with MYC transcriptional activity, both in vitro and in vivo (5).
The first Omomyc-derived compound, OMO-103, successfully completed a phase 1 clinical trial in 2022. Previous results, presented at the 34th EORTC-NCI-AACR Symposium, Barcelona, 26-28 October 2022, showed that this first-in-class MYC inhibitor had few side effects, was tolerable, and stabilized disease in some patients. Patients in the trials had a range of solid tumours including pancreatic, bowel, and non-small cell lung cancers, and had received at least three prior lines of therapy (6).
Lasting structural integrity of therapeutic proteins in the target tissue is crucial to maintain their proper function. Since the in vivo stability of these agents can be affected by proteolytic degradation, the validity of Omomyc as the first direct inhibitor of MYC has frequently been questioned (8). A recently published study demonstrated, for the first time, that Omomyc behaves as a stable protein in tumour tissue, with longer lasting structural integrity compared to in blood (7).
”Our findings are especially relevant now that Omomyc is being evaluated in clinical trials (Ph1/2 trial) since pharmacokinetic data are usually collected by analysis of blood samples in clinical practice,
Dr. Soucek
Conclusion:
A study published in the journal Genes & Development showed that the expression of Omomyc in preclinical melanoma models disrupts MYC activity and alters gene expression profiles, reducing cancer proliferation and progression (10). This suggests that MYC-targeted therapy by Omomyc could potentially open up a new treatment avenue for melanoma and points to the future development of clinical trials to assess the efficacy of this mini-protein against this tumour type (8). Excitingly, Dr. Laura Soucek’s group has also demonstrated the antimetastatic capacity of Omomyc for the first time in breast cancer (11).
In addition to the Omomyc mini-protein, Dr. Soucek’s laboratory also developed a monoclonal antibody targeting Omomyc, which can be used to study Omomyc levels, as well as better understand its function and effect on biological processes of interest, including cancer.
Omomyc: promising results seen in clinical trials