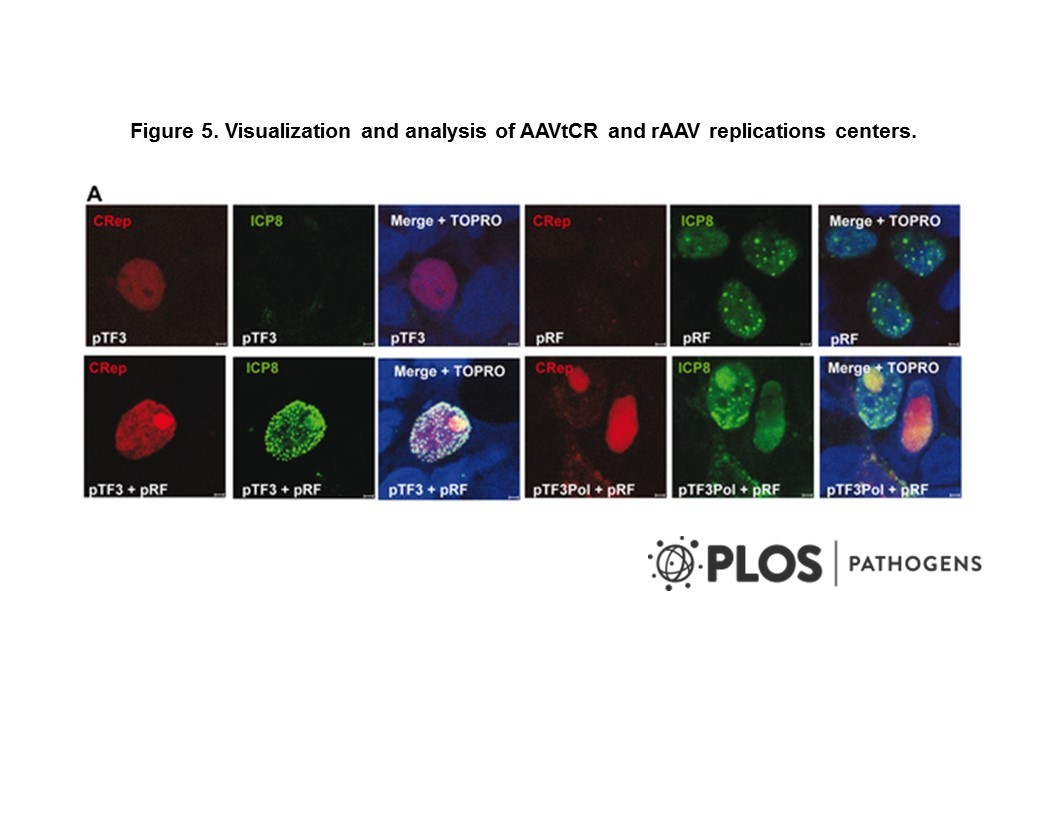
Cat. #162094
Coming soon HCI-028 PDX
Cat. #: 162094
Cancer Type: Breast Cancer
Cancer Sub-type: Infiltrating Ductal Carcinoma
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Alana L Welm, Yi-Chun Lin, Yoko Sakata DeRose
Institute: The University of Utah Research Foundation
Primary Citation: Guillen, et al. 2022. Nature Cancer. Feb; 3(2):232-250. PMID: 35221361
Tool Details
*FOR RESEARCH USE ONLY
- Research area: Breast Cancer
- Description: Please register your interest through the enquiry button (quote not currently available)
Human breast cancer-derived xenograft that retains high fidelity to original tumour and provides valuable resources for drug discovery and precision oncology. This panel of Patient Derived Xenografts provide models for some of the deadliest forms of breast cancer including drug-resistant, metastatic tumours, and endocrine-resistant estrogen receptor-positive (ER+) and HER2+ tumours.
Sample collected in 2016 from pleural effusion of Caucasian female, age 31 at time of collection with a primary diagnosis of IDC; 2010. Patient was a former smoker for 10 years, and had clinical metastasis detected in bone, lung, ovary, liver, and brain. Patient had undergone radiation therapy of breast in 2011 and had received systemic treatment of doxorubicin, cyclophosphamide, paclitaxel 2011; tamoxifen 2011; metformin trial 2014-2015; letrazole 2015; zolendronic acid 2015 (1 dose); pertuzumab, trastuzumab, taxotere 2015; everolimus and exemestane2015; capecitabine, lapatinib 2015;docetaxel 2016; pertuzumab, trastuzumab 2016; ado-trastuzumab emtansine 2016; methotrexate 2016; topotecan 2016 prior to sample collection. Patient and PDX characteristics were as follows - ER status: negative, PR status: negative, HER2 status: negative. PDX information: PAM50 subtype is HER2-enriched and metastasis in lymph node, lung, and liver detected.
Patient Details
- Cancer type: Breast Cancer
- Cancer subtype: Infiltrating Ductal Carcinoma
- Biopsy site: Pleural Effusion Fluid
- Gender: Female
- Patient ethnicity: Caucasian
- Treatment history: Pretreated: Patient had undergone radiation therapy of breast in 2011 and had received systemic treatment of doxorubicin, cyclophosphamide, paclitaxel 2011; tamoxifen 2011; metformin trial 2014-2015; letrazole 2015; zolendronic acid 2015 (1 dose); pertuzumab, trastuzumab, taxotere 2015; everolimus and exemestane2015; capecitabine, lapatinib 2015; docetaxel 2016; pertuzumab, trastuzumab 2016; ado-trastuzumab emtansine 2016; methotrexate 2016; topotecan 2016 prior to sample collection
Engraftment Details
- Mice passaged: Yes
- Engraftment site: Cleared mammary fat pad
- Host strain: Immunocompromised mice NOD scid gamma (NSG) Jackson Laboratory 5557; NOD/scid, Jackson Laboratory 1303 or NOD rag gamma (NRG), Jackson Laboratory 7799
- Histology: PAM50 subtype Her2 enriched
- Production details: Fresh or thawed human breast tumour fragments were implanted into the cleared inguinal mammary fat pad of female Immune-compromised mice. For bone metastasis samples, bone fragments were coimplanted. For liquid specimens, pleural effusion, or ascites fluid, 1-2 milion cells were injected into cleared mammary fat pads in Matrigel. For ER+ tumours, mice were dosed with E2 beeswax pellets and given supplemental E2 via drinking water. When tumours reached 1-2 cm in diameter, tumours were aseptically collected and reimplanted into new m ice or banked. Estrogen-independent ER+ breast PDX models were generated when ER+ PDX tumours were transplated into overiectomized mice without E2 supplementation.
Handling
- Initial handling information: Implant into the cleared inguinal mammary fat pad of female Immune-compromised mice NSG and NRG. Both NSG and NRG (fresh) time to growth: 5 months to 2 cm
- Additional notes: Additional Information on PDX establishment: https://www.nature.com/articles/s43018-022-00337-6/figures/9
Application Details
- Genetic data: Whole exome sequencing, SNP array, CNV data and RNA sequence from Guillen et al. 2022 Nature Cancer, is available in NIH database dbGaP under accession number phs002479.v1.p1
References
- Tufail, et al. 2024. Journal of Translational Med. Jan 3:22(1):15. PMID: 38172946
- Bhattacharya, et al. 2023. Journal of Experimental & Clinical Cancer Research. Dec 16:42(1):343. PMID: 38102637
- Prekovic, et al. 2023. EMBO Molecular Medicine. Dec 7:15(12):e17737. PMID: 37902007
- Daneshdoust, et al. 2023. Cells. Sep 30:12(19):2338. PMID: 37830602
- Wang, et al. 2023. Cell Bioscience. Dec 1:13(1):224. PMID: 38041134
- Guillen, et al. 2022. Nature Cancer. Feb 3(2):232-250. PMID: 35221346