Cat. #162275
CRUK0949 T1-R5 TRACERx lung cancer PDX
Cat. #: 162275
Cancer type: Lung cancer
Cancer Sub-type: NSCLC: Lung Squamous Cell Carcinoma
£247.00
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Robert Hynds, David Pearce
Institute: CRUK Lung Cancer Centre of Excellence at University College London
Primary Citation: Hynds R.E. et al., Nature Communications 15, 4653 (2024). PMID: 38821942
Tool Details
*FOR RESEARCH USE ONLY (for other uses, please contact the licensing team)
- Alternate name: CRUK0949_PDX_T1-R5
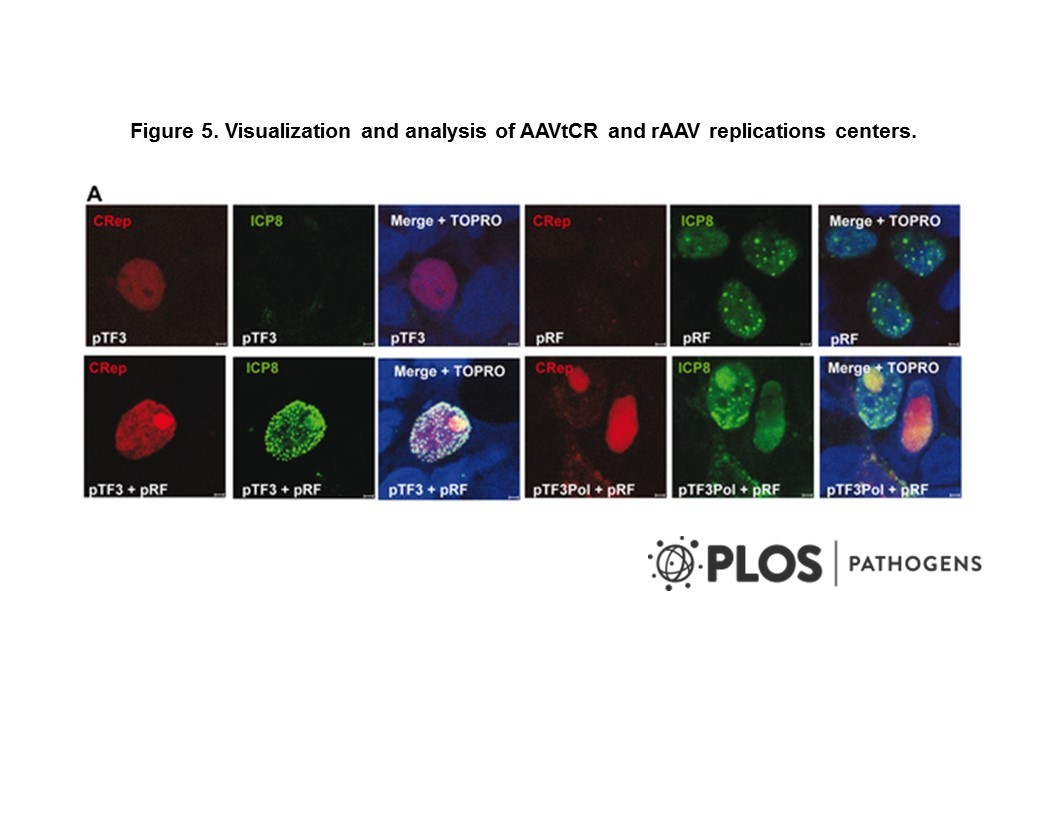
- Description: Human non-small cell lung cancer (NSCLC) patient-derived xenograft (PDX) model generated from primary tumour from patient enrolled in the Lung TRACERx study. PDX models are highly valuable in recapitulating patient tumour characteristics. These include more representative intratumour heterogeneity, genomic features, metastatic patterns and drug responses than traditional cell line and animal models. Therefore, PDX models can be used to support accurate disease modelling and preclinical in vivo drug validation studies. This pioneering collection of TRACERx NSCLC PDX models were derived from multiple regions of primary tumours from patients enrolled in the Lung TRACERx study. This approach significantly increased PDX engraftment success and these models provide valuable insights into how well PDX models represent the intratumour heterogeneity of source patient tumours.
Patient Details
- Cancer type: Lung cancer
- Cancer subtype: NSCLC: Lung Squamous Cell Carcinoma
- Cancer stage grade: 3a
- Biopsy site: Primary lung resection
- Gender: Male
- Age y o : 79
- Patient ethnicity: White-European
- Treatment history: Naive
- Smoking status: Ex-Smoker
- Lung tracerx patient id: CRUK0949
- Patient tumour number: T1
- Primary tumour region: R5
- Driver mutation: Primary tumour clonal mutations- PTEN (clonal - PTEN:NM_000314:exon6:c.537_544del:p.179_182del)
Engraftment Details
- Mice passaged: Yes
- Engraftment site: Subcutaneous (flank)
- Sample type: Minced tumour
- Host strain: NOD scid gamma (NSG) mice
- Production details: Primary NSCLC tumour material was minced and injected subcutaneously in the flank of male NSG mice in growth factor reduced matrigel. To prepare frozen samples for injection, the sample cryovial was warmed in a 37°C water bath until just thawed. The whole sample was then transferred into a sterile 1.5 ml centrifuge tube and topped up with transport medium. The sample was centrifuged at 300 x g for 5 minutes and the supernatant was removed. The sample was then washed with transport medium. If required, any large pieces of tissue were collected and finely minced with a scalpel to ensure they could pass through the needle. The sample was then centrifuged again and gently resuspended in ice-cold Matrigel, and kept on ice before subcutaneous injection. When tumours reached 1.5 cm3 in volume (calculated as 0.5 x length x width2), tumours were aseptically collected and reimplanted into new mice or cryopreserved and banked.
Handling
- Initial handling information: Primary NSCLC tumour material was minced and injected subcutaneously in the flank of male NSG mice in growth factor reduced matrigel.
- Additional notes: Additional information on PDX model establishment and characterisation can be found in the Product Information Sheet.
Application Details
- Genetic data: Mutation data is available from Hynds R.E. et al., Nature Communications 2024. Raw whole-exome sequencing data available via TRACERx data committee under study accession code EGAS00001007364 and dataset accession code EGAD00001012228.
- Application: Whole-exome sequencing, Hematoxylin and eosin staining, Immunohistochemistry
References
- Hynds R.E. et al., Nature Communications 15, 4653 (2024). PMID: 38821942
- Jamal-Hanjani M. et al.,PLoS Biol.12, e1001906 (2014). PMID: 25003560




![Anti-CAR Whitlow Linker [1C3C3]](https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-300x322.jpg 300w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-280x300.jpg 280w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-954x1024.jpg 954w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-768x824.jpg 768w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025.jpg 1193w)