Cat. #160520
Anti-RA015/11.88 [11.88]
Cat. #: 160520
Unit size: 100 ug
Availability: 10-12 weeks
Target: Neutrophil Extracellular Trap Antigen
Class: Recombinant
Application: ELISA ; WB
Host: Human
£300.00
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Institute: Queen Mary University of London
Tool Details
*FOR RESEARCH USE ONLY
- Name: Anti-RA015/11.88 [11.88]
- Alternate name: NET (Neutrophil Extracellular Trap)
- Clone: 11.88
- Class: Recombinant
- Conjugation: Unconjugated
- Host: Human
- Application: ELISA ; WB
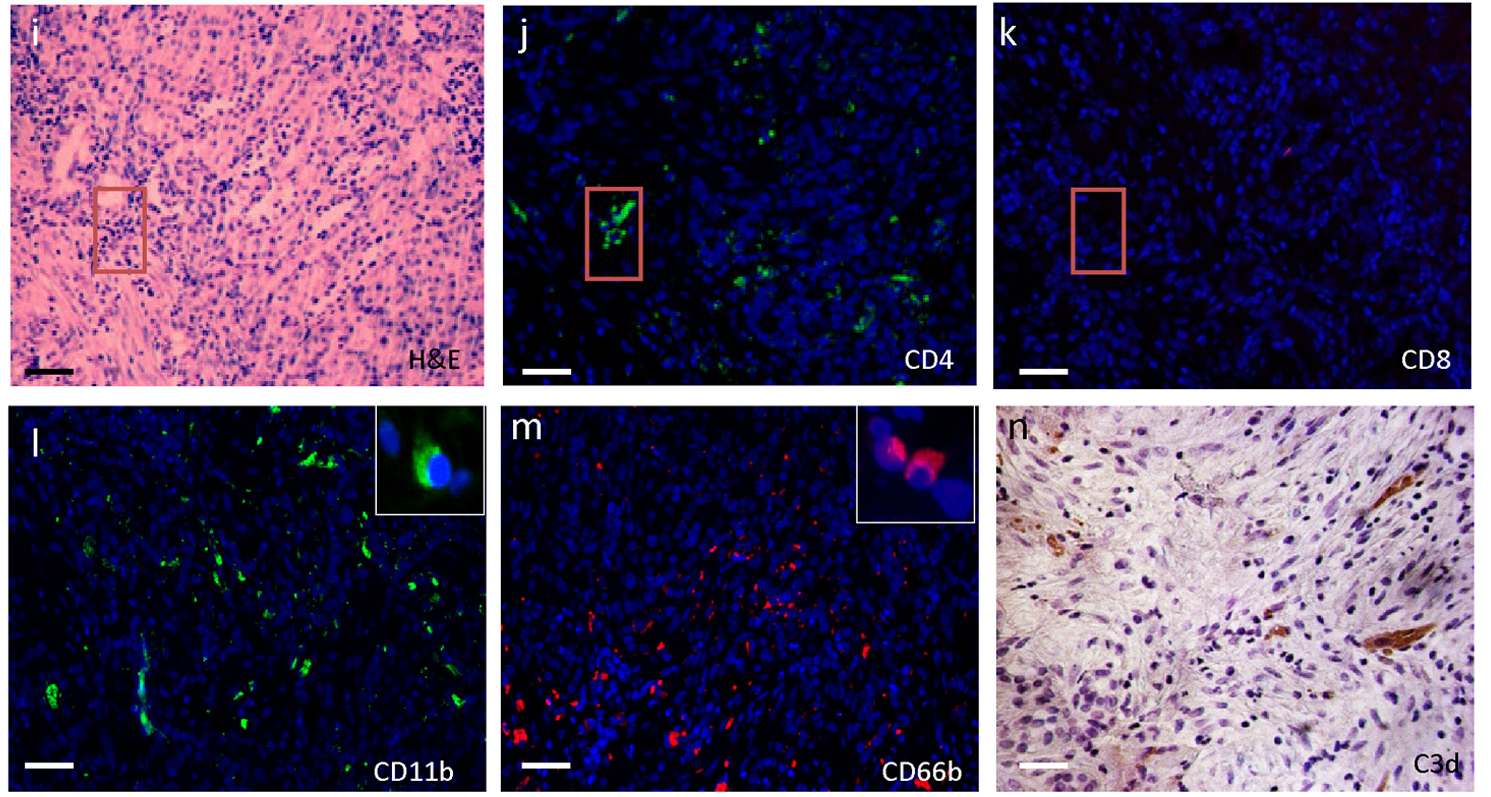
- Description: Rheumatoid arthritis (RA) is a joint-destructive inflammatory disorder characterized by breach of self-tolerance and production of antiÄËĂÂĂÂcit-peptide/protein Abs (ACPA). In the RA synovium, ectopic germinal centers (GCs) support an autoantigen-driven immune response leading to local ACPA+ B cell differentiation (1, 2). Recently, we reported that autoreactive B cells highly mutated within ectopic GCs frequently target cit-histones (cit-H2A/B) contained in neutrophil extracellular trap...
- Immunogen: TBD
- Immunogen uniprot id: TBD
Target Details
- Target: Neutrophil Extracellular Trap Antigen
- Target background: Rheumatoid arthritis (RA) is a joint-destructive inflammatory disorder characterized by breach of self-tolerance and production of antiÄËĂÂĂÂcit-peptide/protein Abs (ACPA). In the RA synovium, ectopic germinal centers (GCs) support an autoantigen-driven immune response leading to local ACPA+ B cell differentiation (1, 2). Recently, we reported that autoreactive B cells highly mutated within ectopic GCs frequently target cit-histones (cit-H2A/B) contained in neutrophil extracellular trap...
Applications
- Application: ELISA ; WB
Handling
- Format: Liquid
- Unit size: 100 ug
- Shipping conditions: Shipping at 4° C
References
- Corsiero et al. 2020. J Immunol. 204(9):2374-2379. PMID: 32221039.