Cat. #162439
Anti-CAR Whitlow Linker [1B4A1]
Cat. #: 162439
Unit size: 1 mg
Target: Chimeric antigen receptor Whitlow scFv peptide linker
Class: Monoclonal
Application: Flow cytometry, IHC, WB
Host: Mouse
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Erik Kimble, Jocelyn Wright
Institute: Fred Hutchinson Cancer Center
Primary Citation: Kimble et al. 2025.J Immunother Cancer. 2025 Nov 18;13(11):e013123. PMID: 41253498
Tool Details
*FOR RESEARCH USE ONLY (for other uses, please contact the licensing team)
- Name: Anti-CAR Whitlow Linker [1B4A1]
- Clone: 1B4A1
- Class: Monoclonal
- Conjugation: Unconjugated
- Host: Mouse
- Application: Flow cytometry, IHC, WB
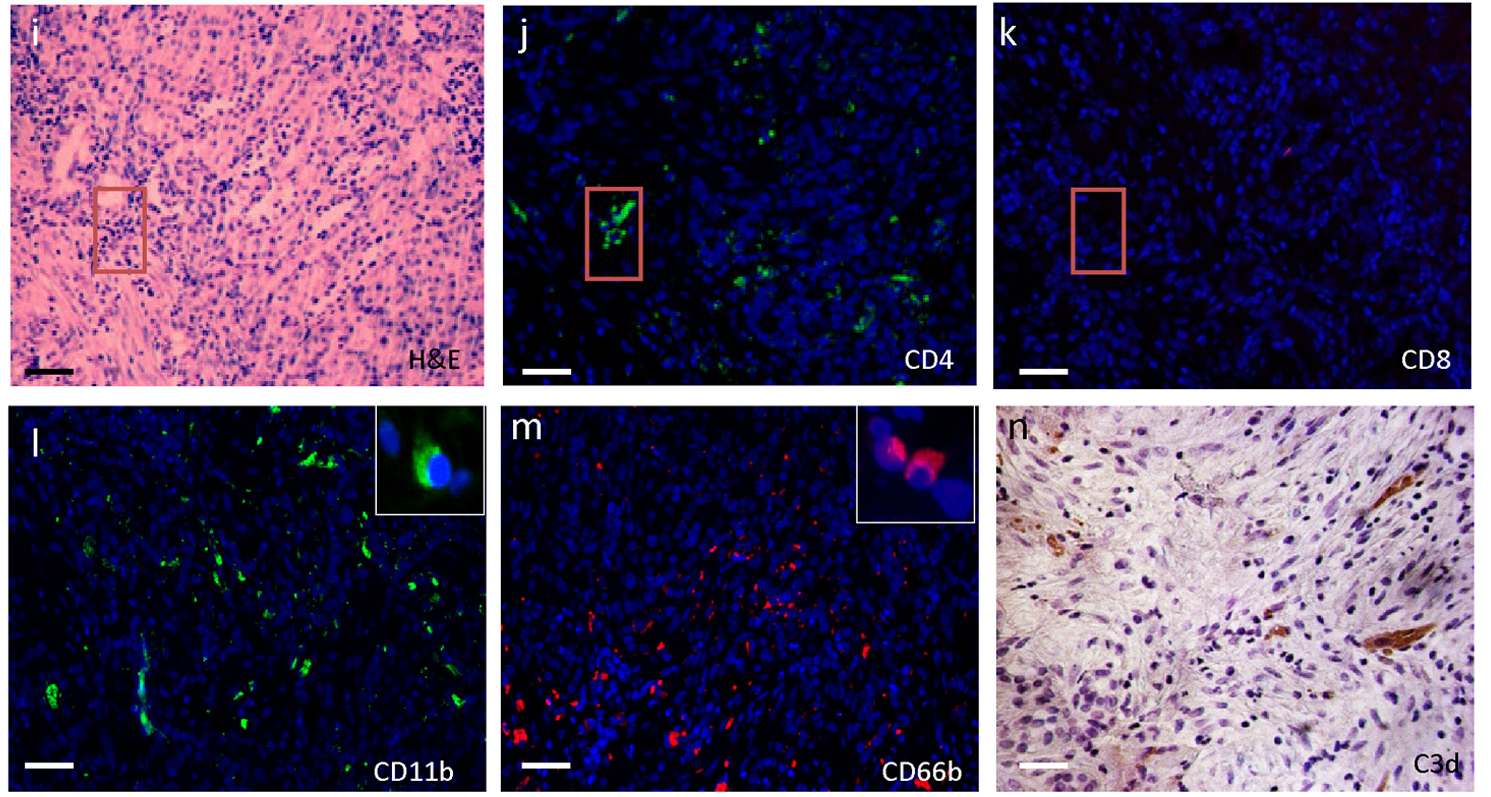
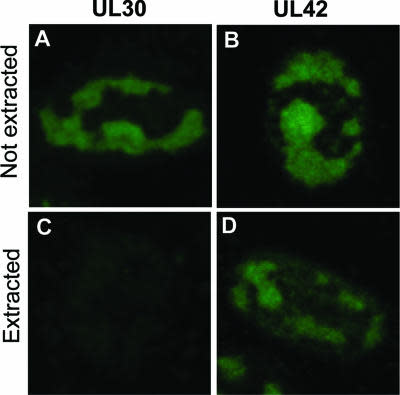
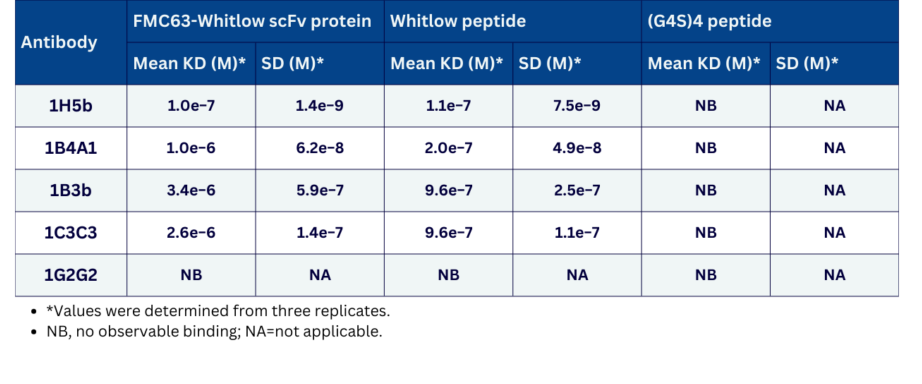
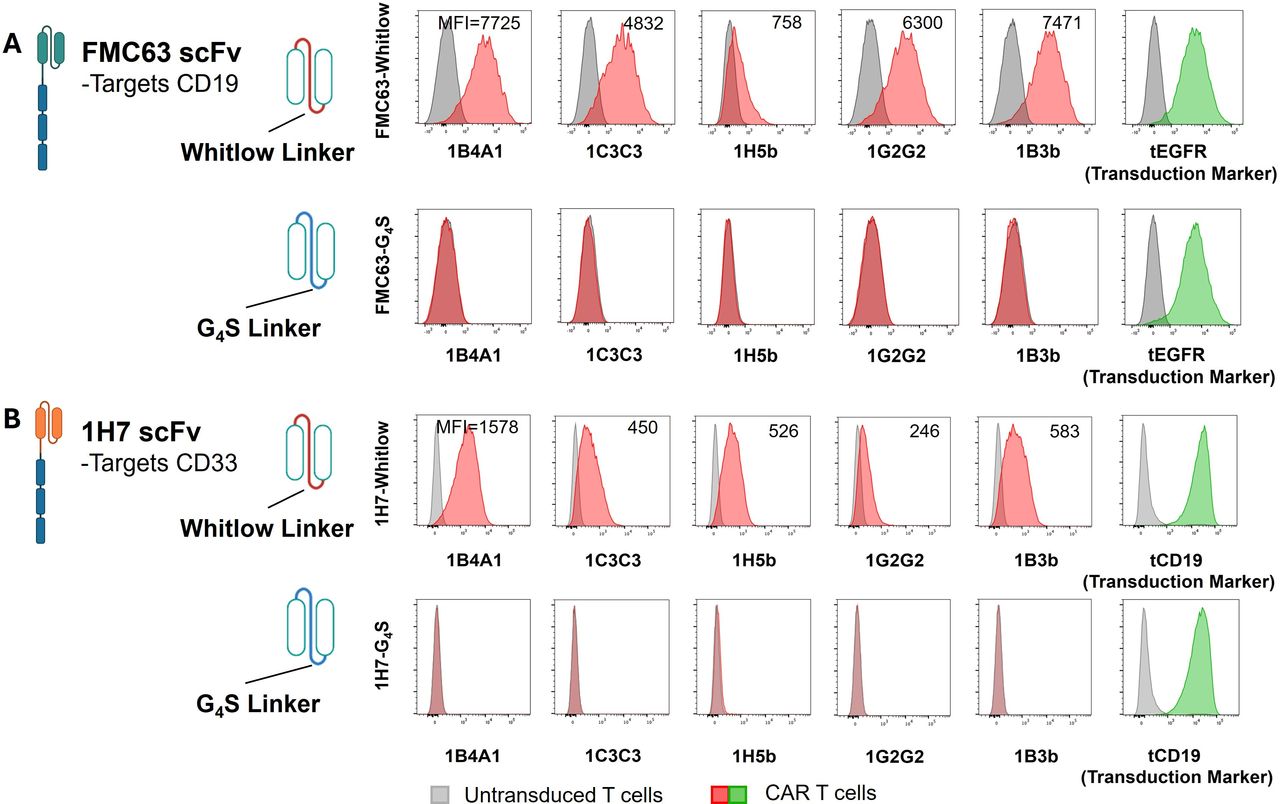
- Description: The anti-CAR Whitlow linker peptide 1H5b clone has been developed to detect cells expressing Whitlow linker-containing CARs with different antigen specificities, including those harbouring the widely employed anti-CD19 FMC63-derived scFv as well as other scFvs, such as those targeting B-cell maturation antigen (BCMA) or CD33. It has been demonstrated to have a mean KD of 0.2 to 1 µM when tested for binding kinetics against FMC63-Whitlow scFv protein and the Whitlow peptide (no binding to G4S4 peptide was found). 1B4A1 antibody has also been demonstrated to stain engineered T cells expressing 1H7- or FMC63-Whiltow, but not 1H7- or FMC63- G4S4 by FACS analysis. This clone has also been shown to detect commercial CAR T cells in the blood of patients undergoing treatment with CAR T cells containing a Whitlow linker (liso-cel, axi-cel, brexu-cel, ide-cel) but not those being treated with CAR T cells without a Whitlow linker (tisa-cel and cita-cel). Finally, 1B4A1 is able to detect CAR T cells with a Whitlow linker in situ in archival biopsies from patients being treated with commercial CAR T cell therapy (liso-cel and axi-cel).
- Immunogen: Synthetic Whitlow peptide
- Isotype: IgG2a
Target Details
- Target: Chimeric antigen receptor Whitlow scFv peptide linker
- Target background: While CAR T-cell therapies have revolutionized the treatment of B-lineage malignancies, high-resolution tracking of CAR-engineered cells within the tumor microenvironment (TME) remains a significant technical challenge, particularly in archival formalin-fixed paraffin-embedded (FFPE) tissues. To address this, murine monoclonal antibodies (mAbs) have been developed to specifically target the Whitlow linker, a synthetic peptide commonly utilized in the scFv domains of multiple FDA-approved CAR products (e.g., axi-cel, liso-cel). Because this linker is absent in native human tissue and conserved across various antigen specificities, these mAbs provide a universal tool for the in situ identification, selection, and functional analysis of CAR-expressing cells. This methodology enables a more precise evaluation of CAR T-cell infiltration, persistence, and correlation with clinical outcomes or toxicities.
Applications
- Application: Flow cytometry, IHC, WB
Handling
- Unit size: 1 mg

![Anti-CAR Whitlow Linker [1B4A1]](https://cancertools.org/wp-content/uploads/Figure-5-Kimble-et-al.-J-Immunother-Cancer-2025.jpg)
![Anti-CAR Whitlow Linker [1B4A1] - Image 4](https://cancertools.org/wp-content/uploads/Figure-3-Kimble-et-al.-J-Immunother-Cancer-2025.jpg)
![Anti-CAR Whitlow Linker [1C3C3]](https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-300x322.jpg 300w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-280x300.jpg 280w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-954x1024.jpg 954w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-768x824.jpg 768w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025.jpg 1193w)

![Anti-CAR Whitlow Linker [1B4A1]](https://cancertools.org/wp-content/uploads/Figure-5-Kimble-et-al.-J-Immunother-Cancer-2025-300x396.jpg 300w, https://cancertools.org/wp-content/uploads/Figure-5-Kimble-et-al.-J-Immunother-Cancer-2025-227x300.jpg 227w, https://cancertools.org/wp-content/uploads/Figure-5-Kimble-et-al.-J-Immunother-Cancer-2025-776x1024.jpg 776w, https://cancertools.org/wp-content/uploads/Figure-5-Kimble-et-al.-J-Immunother-Cancer-2025-768x1013.jpg 768w, https://cancertools.org/wp-content/uploads/Figure-5-Kimble-et-al.-J-Immunother-Cancer-2025.jpg 970w)