Cat. #162440
Anti-CAR Whitlow Linker [1B3b]
Cat. #: 162440
Unit size: 1 mg
Target: Chimeric antigen receptor Whitlow scFv peptide linker
Class: Monoclonal
Application: Flow cytometry, IHC
Host: Mouse
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Erik Kimble, Jocelyn Wright
Institute: Fred Hutchinson Cancer Center
Primary Citation: Kimble et al. 2025.J Immunother Cancer. 2025 Nov 18;13(11):e013123. PMID: 41253499
Tool Details
*FOR RESEARCH USE ONLY (for other uses, please contact the licensing team)
- Name: Anti-CAR Whitlow Linker [1B3b]
- Clone: 1B3b
- Class: Monoclonal
- Conjugation: Unconjugated
- Host: Mouse
- Application: Flow cytometry, IHC
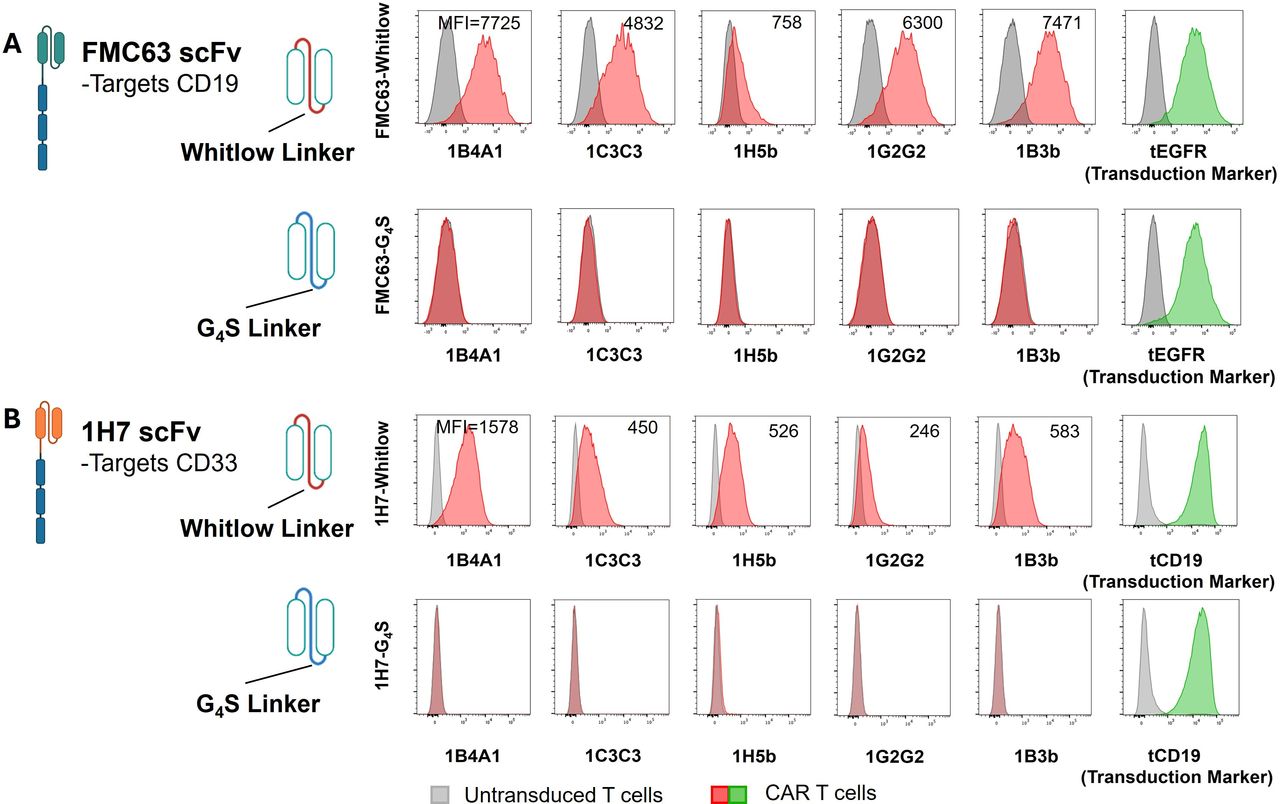
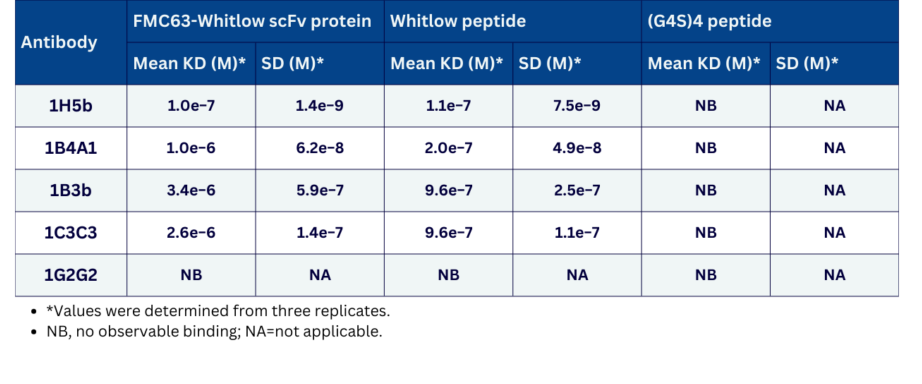
- Description: The anti‑CAR Whitlow linker peptide 1B3b clone has been developed to detect cells expressing Whitlow linker‑containing CARs with different antigen specificities, including those harbouring the widely employed anti‑CD19 FMC63‑derived scFv as well as other scFvs, such as those targeting B‑cell maturation antigen (BCMA) or CD33. It has mean K_D values of approximately 3.4 µM for FMC63‑Whitlow scFv protein and 0.096 µM for the Whitlow peptide, with no observable binding to the G4S4 peptide. In addition, the 1B3b antibody has been demonstrated to stain engineered T cells expressing 1H7‑ or FMC63‑Whitlow, but not 1H7‑ or FMC63‑G4S4 by FACS analysis.
- Immunogen: Synthetic Whitlow peptide
- Isotype: IgG2a
Target Details
- Target: Chimeric antigen receptor Whitlow scFv peptide linker
- Target background: While CAR T-cell therapies have revolutionized the treatment of B-lineage malignancies, high-resolution tracking of CAR-engineered cells within the tumor microenvironment (TME) remains a significant technical challenge, particularly in archival formalin-fixed paraffin-embedded (FFPE) tissues. To address this, murine monoclonal antibodies (mAbs) have been developed to specifically target the Whitlow linker, a synthetic peptide commonly utilized in the scFv domains of multiple FDA-approved CAR products (e.g., axi-cel, liso-cel). Because this linker is absent in native human tissue and conserved across various antigen specificities, these mAbs provide a universal tool for the in situ identification, selection, and functional analysis of CAR-expressing cells. This methodology enables a more precise evaluation of CAR T-cell infiltration, persistence, and correlation with clinical outcomes or toxicities.
Applications
- Application: Flow cytometry, IHC
Handling
- Unit size: 1 mg


![Anti-CAR Whitlow Linker [1C3C3]](https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-300x322.jpg 300w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-280x300.jpg 280w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-954x1024.jpg 954w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025-768x824.jpg 768w, https://cancertools.org/wp-content/uploads/Figure-6-Kimble-et-al.-J-Immunother-Cancer-2025.jpg 1193w)