Cat. #152547
MCF7/LetR-1 Cell Line
Cat. #: 152547
Sub-type: Continuous
Unit size: 1x10^6 cells / vial
Organism: Human
Tissue: Breast
Disease: Cancer
£800.00
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Anne Lykkesfeldt
Institute: Danish Cancer Society
Tool Details
*FOR RESEARCH USE ONLY
- Name: MCF7/LetR-1 Cell Line
- Tool sub type: Continuous
- Parental cell: MCF7
- Organism: Human
- Tissue: Breast
- Disease: Cancer
- Growth properties: Breast cancer cell line resistant to the aromatase inhibitor letrozole. Estrogen receptor positive. Progesterone receptor positive when grown in medium without letrozole.
- Conditional: No
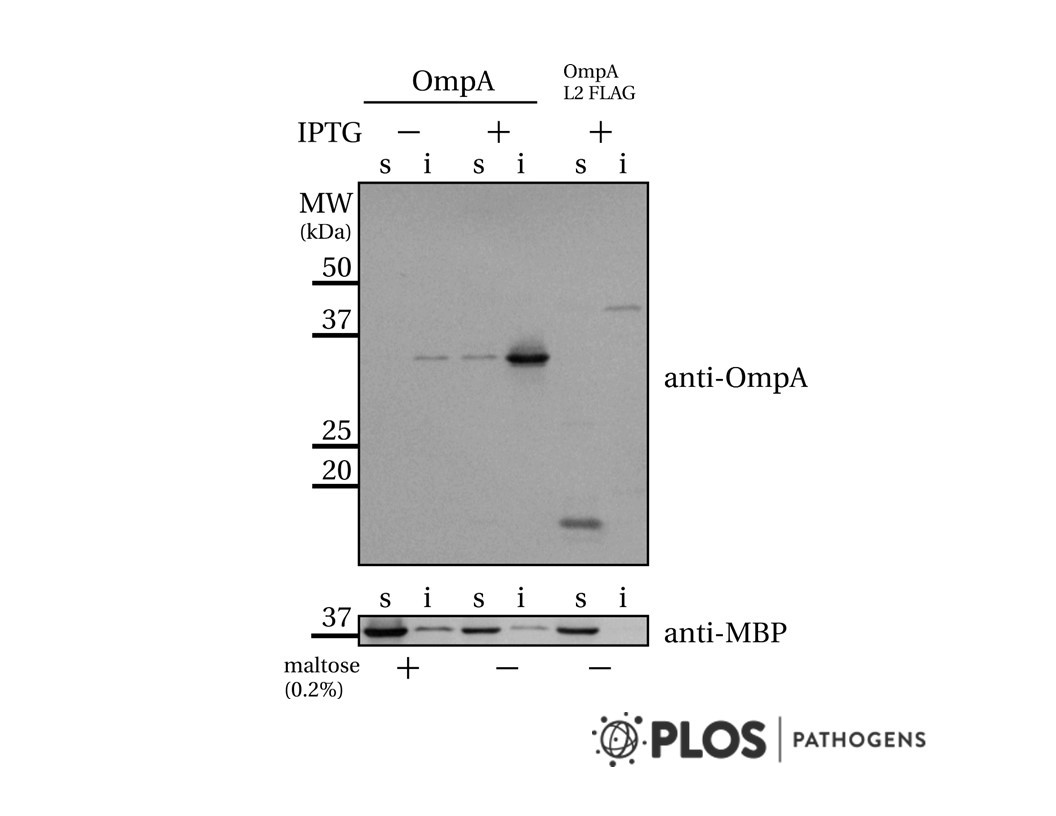
- Description: The MCF7/LetR-1 Cell Line was developed as a model of resistance to anti-cancer treatment with aromatase inhibitors. Third generation aromatase inhibitors (AIs) have proven to be effective treatment for estrogen receptor positive (ER+) breast cancer and are today recommended as first line endocrine therapy for postmenopausal ER+ breast cancer patients, making up the majority of breast cancer patients. However, a major problem is development of resistance against AIs. Since molecular mechanisms of AI resistance are largely undisclosed, the development of cell lines resistant to the non-steroidal AI letrozole allows the study of the molecular basis for resistance to AIs to unravel new targets for treatment.
- Production details: Letrozole-resistant cell lines were established from MCF-7 cells grown in medium with 10% NCS and 10ÄË?Â?Â7 M testosterone. A culture of MCF-7 cells were treated with 10ÄË?Â?Â6 M letrozole for one week, trypsinized and seeded in serial dilutions in 24-well plates. Single colonies were transferred to new wells and gradually expanded in medium with letrozole. After ~2ÄË?Â?Â3 months, the isolated colonies gave rise to letrozole-resistant cell lines, which could be grown in letr...
- Cellosaurus id: CVCL_5A17
Target Details
- Target: Letrozole resistant
Applications
- Application notes: Human breast cancer cell line derived from MCF-7 cells Other related cell lines: - LetR-2, LetR-3 and LetR-4 resistant to the non-steroidal AI letrozole - ExeR-1, ExeR-2, ExeR-3 and ExeR-4 resistant to the steroidal AI exemestane - AnaR-1, AnaR-2, AnaR-3 and AnaR-4 resistant to the non-steroidal AI anastrozole Passage 436 (AL3117. AL3118)
Handling
- Format: Frozen
- Passage number: Passage 436 (AL3117. AL3118)
- Growth medium: Phenol-red-free DMEM/F12 medium supplemented with 10% newborn calf serum, 2.5 mM Glutamax, 6 ng/ ml insulin, 0.1 uM testosterone and 1 uM letrozole.
- Unit size: 1x10^6 cells / vial
- Shipping conditions: Dry ice
Related Tools
- Related tools: MCF7/LetR-2 Cell Line ; MCF7/LetR-4 Cell Line ; MCF7/LetR-3 Cell Line Other related cell lines: - LetR-2, LetR-3 and LetR-4 resistant to the non-steroidal AI letrozole - ExeR-1, ExeR-2, ExeR-3 and ExeR-4 resistant to the steroidal AI exemestane - AnaR-1, AnaR-2, AnaR-3 and AnaR-4 resistant to the non-steroidal AI anastrozole
References
- Hole et al. 2015. Breast Cancer Res Treat. 149(3):715-26. PMID: 25667100.
- Hole et al. 2015. Int J Oncol. 46(4):1481-90. PMID: 25625755.
- Aurora kinase A and B as new treatment targets in aromatase inhibitor-resistant breast cancer cells.
- New cell culture model for aromatase inhibitor-resistant breast cancer shows sensitivity to fulvestrant treatment and cross-resistance between letrozole and exemestane.